Records of Natural Products
Year: 2020 Volume: 14 Issue:3 May-June
1) New Aromadendrane Sesquiterpenoid Pseuboydone F from the Marine-derived Fungus Pseudallescheria boydii F44-1
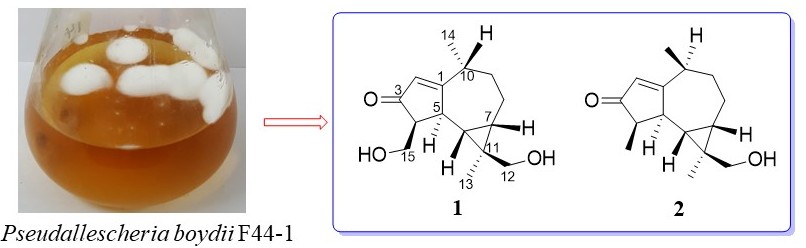
A new aromadendrane sesquiterpenoid pseuboydone F (1), along with a known pseuboydone A (2), were isolated from the marine-derived fungus Pseudallescheria boydii F44-1 associated with the soft coral Sarcophyton sp.. The structures were elucidated by HRMS, 1D and 2D NMR spectroscopic data.
DOI http://doi.org/10.25135/rnp.152.19.07.1343 Keywords Marine fungus Pseudallescheria boydii aromadendrane sesquiterpenoid pseudboydone F DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.2) A New Sesquiterpene from Schisandra sphenanthera
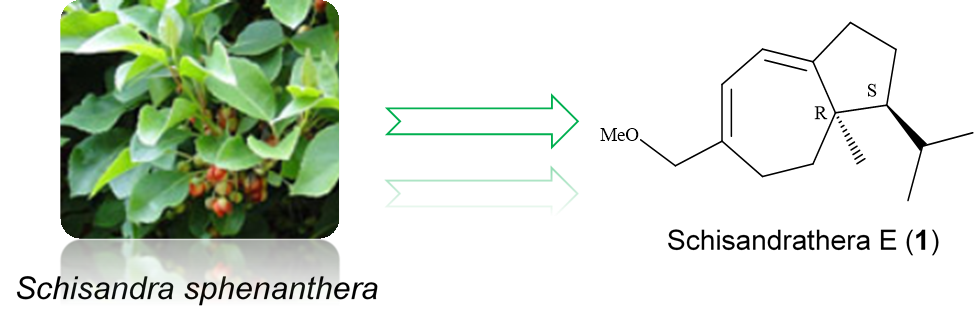
A new sesquiterpene, schisandrathera E (1) and seven dibenzocyclooctadiene lignans as schisantherin D (2), schisantherin B (3), tigloylgomisin P (4), schisphenin E (5), angeloylgomisin H (6), (+)-deoxyschizandrin (7), and (+)-gomisin K3 (8) were isolated from the leaves of Schisandra sphenanthera Rehder & E.H.Wilson. Their structures were elucidated by spectroscopic and mass spectrometric analyses, including 1D-, 2D-NMR, HR-ESI-MS and ECD spectra. Compound 1 displayed moderate cytotoxicity against both PC3 and MCF4 cell lines in MTT assay with IC50 values of 22.60 and 7.80 µM, respectively.
DOI http://doi.org/10.25135/rnp.153.19.09.1406 Keywords Schisandra sphenanthera schisandrathera E sesquiterpene dibenzocyclooctadiene DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.3) α-Pinene, Caryophyllene and β-Myrcene from Peucedanum terebinthaceum Essential oil: Insecticidal and Repellent Effects on Three Stored-Product Insects

The aim of this work was to evaluate the bioactivities of Peucedanum terebinthaceum (Fisch.) Fisch. ex Turcz. essential oil and its three second rich constituents against Tribolium castaneum Herbst, Lasioderma serricorne Fabricius and Liposcelis bostrychophila Badonnel. The essential oil from aerial part of P. terebinthaceum was obtained by hydrodistillation and analyzed by gas chromatography-mass spectrometry. Thirty-three constituents were confirmed by GC-MS, which accounting for 91.5% of the total oil. The principal constituents included β-thujene (21.4%), β-terpinene (11.8%), germacrene D (9.4%) and dihydro-cis-α-copaene-8-ol (8.0%). Besides, β-myrcene (6.2%), Linalyl isovalerate (4.3%), α-pinene (4.0%), caryophyllene (3.6%), (Z)-α-farnesene (3.6%) and β-elemene (3.0%) were also detected in relatively lower content. The essential oil possessed promising potential in pest control, as it showed strong contact toxicity and repellent effects on T. castaneum and L. serricorne. Three major constituents α-pinene, caryophyllene and β-myrcene were toxic to three insect species in contact assays and showed repellent effects on T. castaneum and L. bostrychophila. This work revealed the insecticidal capacity of P. terebinthaceum and would provide some information for the development of new strategies in the control of insect pests.
DOI http://doi.org/10.25135/rnp.149.19.05.1287 Keywords Fumigant toxicity Contact toxicity Tribolium castaneum Herbst Lasioderma serricorne Fabricius Liposcelis bostrychophila Badonnel DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.4) Diterpenoids and Sesquiterpenoids from Syzygium fluviatile
Abstract: A new monocyclic diterpenoid, cassipouryl formate, (1) together with three known diterpenoids (2−4) and five known sesquiterpenoids (5−9) were isolated from the twigs and leaves of Syzygium fluviatile. The new isolate was elucidated by various spectroscopic technologies. This was the first example of the chemical constituents from this species and the first report on the diterpenoids from genus Syzygium.
DOI http://doi.org/10.25135/rnp.158.19.08.1393 Keywords Syzygium fluviatile Myrtaceae diterpenoids sesquiterpenoids. DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.5) Two New 2(1H)-Pyrazinone Derivatives from the Plant Endophyte Streptomyces sp. KIB-H1992

Two new 2(1H)-pyrazinone derivatives, 3,6-diisopropyl-5-methylpyrazin-2(1H)-one (1) and 5-(hydroxymethyl)-3,6-diisopropylpyrazin-2(1H)-one (2), were isolated from the fermentation broth of endophytic actinomycete Streptomyces sp. KIB-H1992. Their structures were established based on the detailed spectroscopic analyses, including ESI-MS, HR-ESI-MS, 1D- and 2D NMR spectra.
DOI http://doi.org/10.25135/rnp.154.1907.1355 Keywords 2(1H)-pyrazinone derivatives endophytic actinomycete Streptomyces sp. spectroscopic analyses DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.6) Mosquito Larvicidal Activity on Aedes albopictus and Constituents of Essential Oils from Manglietia dandyi (Gagnep.) Dandy
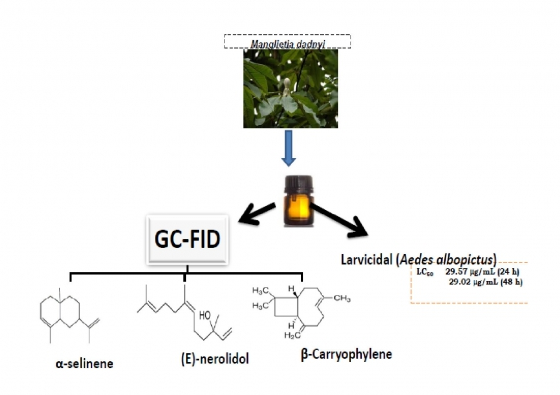
Herein, we report the results of our investigation on essential oils obtained by hydrodistilation of the leaves and fruits of Manglietia dandyi (Gagnep.) Dandy. The constituents of the oils were analyzed by gas chromatography-flame ionization detector (GC-FID) and gas chromatography-mass spectrometry (GC-MS). The yields of the oils were 0.25% and 0.21% (v/w, leaf and fruit respectively), calculated on a dry weight basis. The main constituents of the leaf oils were (E)-nerolidol (18.4%) and α-selinene (11.0%) while the fruit oils contained abundance of β-caryophylene (27.7%), d-cadinene (13.7%), α-humulene (13.2%) and α-copaene (11.6%). The results indicated that M. dandyi leaf oil exhibited 96% and 100% mortality towards the fourth-instant larvae of Aedes albopictus at 24 h at tested concentrations of 50 µg/mL and 100 µg/mL. The oil only displayed 100% mortality at concentrations of 50 and 100 µg/mL and under 48 h. The minimum lethal concentrations, LC50, were 29.57 mg/mL (24 h) and 29.02 mg/mL (48 h); while the LC90 values of 46.21 mg/mL and 42.29 mg/mL were obtained respectively at 24 h and 48 h mg/mL. The chemical constituents and larvicidal action of M. dandyi essential oils are being reported for the first time.
DOI http://doi.org/10.25135/rnp.151.19.07.1325 Keywords Manglietia dandyi essential oil sesquiterpenes larvicidal activity Aedes albopictus DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.7) A New Sesquiterpene and Known Alkaloids from Toddalia asiatica and Their Inhibitions Against Phosphodiesterase-4
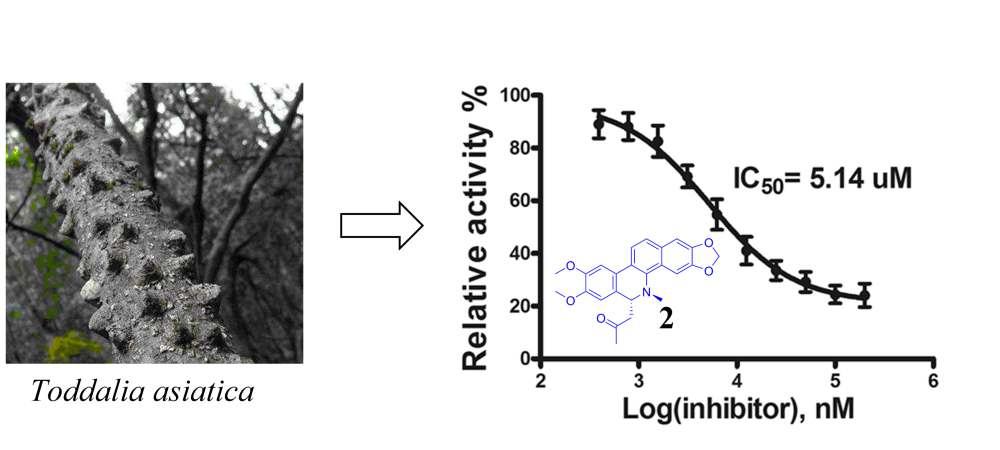
A new sesquiterpene (1) and nine known alkaloids (2-10) were isolated from the roots of Toddalia asiatica. The structure of compound 1 were resolved by NMR and HRESI data, as well as ECD calculation for determining the absolute configuration. The known compounds were identified to be 8-acetonyldihydronitidine (2), 8-acetonyldihydroavicine (3), dihydronitidine (4), oxynitidine (5), decarine (6), skimmianine (7), g-fagarine (8), N-methylflindersine (9), and 4-methoxy-N-methyl-2-quinolone (10) by comparing the NMR data with those in the literature. Compound 1 is the first eremophilane-type sesquiterpenoid isolated from this species. The known compounds 2, 3, and 6 were discovered for the first time from the genus Toddalia. All the isolated compounds were evaluated for their inhibitory effects against phosphodiesterase-4, as result, compound 2 showed strong inhibitory effect against phosphodiesterase-4 with an IC50 value of 5.14 mM.
DOI http://doi.org/10.25135/rnp.157.1907.1356 Keywords Toddalia asiatica sesquiterpene alkaloids phosphodiesterase-4 DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.8) Antimicrobial Effect and Antioxidant Activity of Triterpenes Isolated from Gymnema sylvestre R. Br.
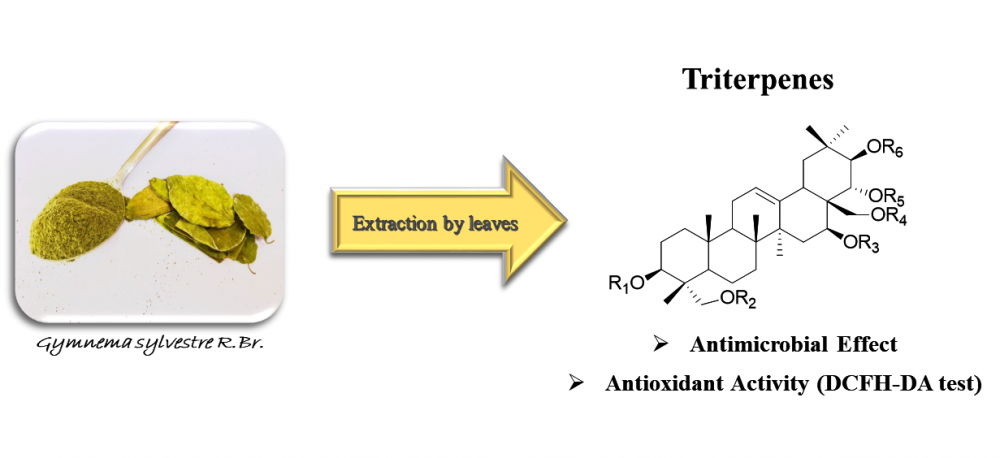
Gymnema sylvestre is a commonly used herb in Ayurvedic medicine. The demand for its extracts in the commercial and pharmaceutical fields has been steadily increasing in recent years. Its extracts are used to treat various ailments as well as for their antimicrobial properties. This study has evaluated the antimicrobial effects of different G. sylvestre extracts and of eight triterpenes isolated from the most active extract on six bacterial poultry pathogens i.e. Bacillus subtilis, Enterococcus faecalis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Enterobacter aerogenes. In particular, it has been evaluated the minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of all extracts and isolated triterpenes. Finally, the cytotoxicity activity of triterpenes was evaluated by MTT assay and their antioxidant activity in basal and oxidant conditions by DCFH-DA assay.
DOI http://doi.org/10.25135/rnp.165.19.10.1448 Keywords Gymnema sylvestre antimicrobial effects triterpenes gymnemic acids herbal drug DCFH-DA assay DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.9) Chemical Composition, Antibacterial and Cytotoxic Activities of the Essential Oil from Ficus tikoua Bur.

The chemical composition and biological activities of the essential oil from Ficus tikoua Bur. were reported for the first time. Fifty-three compounds, accounting for 99.60% of the total essential oil composition, were identified and the main components were palmitic acid (51.13%) and linoleic acid (47.54%). The essential oil revealed significant antibacterial activity with the inhibition zones (7.89–10.59 mm), MIC (0.20–6.25 mg/mL) and MBC (0.20–12.50 mg/mL) against Staphylococcus aureus, Bacillus subtilis, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa and Proteus vulgaris. The essential oil exhibited significant cytotoxicity against A549, NCI-H1299, PC-3 and K562 tumor cells with the IC50 values of 131.08, 50.32, 120.58 and 31.68 μg/mL, respectively. The essential oil exhibited selective cytotoxic activity to human tumor cell lines, with a significantly lower cytotoxicity to human normal cell line (MRC-5, IC50 = 161.75 μg/mL) than to tumor cells. Additionally, palmitic acid, as the major compound, aslo revealed significant antibacterial and cytotoxic activities.
DOI http://doi.org/10.25135/rnp.161.19.10.1450 Keywords Ficus tikoua Bur. essential oil GC-MS palmitic acid antibacterial activity cytotoxic activity DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.10) Steroidal Components from the Roots and Rhizomes of Smilacina henryi and Their Cytotoxic Activities
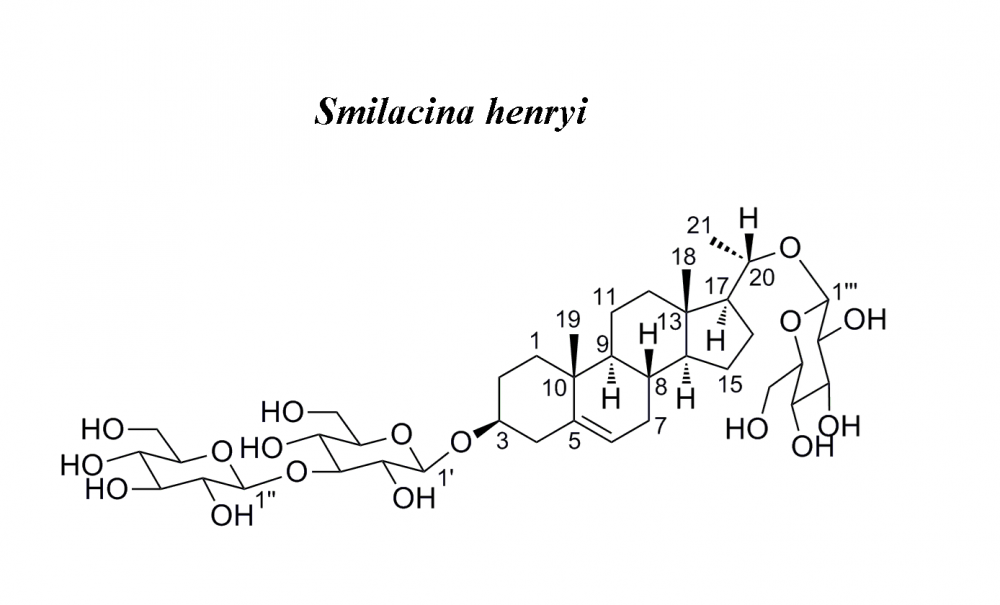
Nine steroidal components, including a new pregnane glycoside (1), were obtained from the roots and rhizomes of Smilacina henryi. Their structures were determined via extensive spectroscopic data including, IR, HRESIMS and 1D, 2D NMR data analysis. Furthermore, their cytotoxic activities against human HepG2 and SW620 tumor cells were evaluated by the MTT method and compounds 2, 3, 5, 8 and 9 showed moderate activity with IC50 values raging from 18.4 to 86.3 μM.
DOI http://doi.org/10.25135/rnp.156.1908.1390 Keywords Smilacina henryi steroidal components structure identifition cytotoxicity DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.