Records of Natural Products
Year: 2025 Volume: 19 Issue:1 January-February
1) Editorial
2) Scleromitrion diffusum:A Comprehensive Review of its Botany, Phytochemistry, Pharmacology and Clinical Application
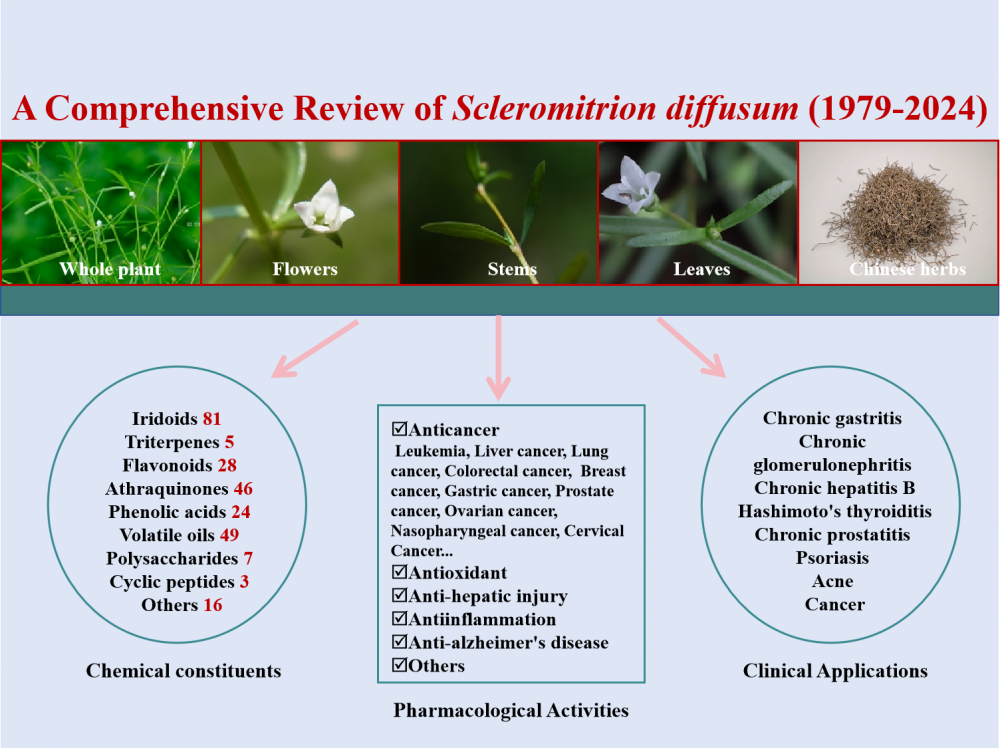
Scleromitrion diffusum (SD) is a medicinal plant belonging to the family Rubiaceae, primarily distributed across Asia. It has a long-standing history of use in Traditional Chinese Medicine (TCM) as an antipyretic-detoxicate agent. This paper reviews studies conducted between 1979 and 2024 that encompass the phytochemistry, pharmacology, and clinical applications of SD. To date, over 259 compounds have been identified from SD, including iridoids, triterpenes, flavonoids, anthraquinones, phenolic acids, essential oils, polysaccharides, and cyclic peptides. Pharmacological investigations indicate that the compounds and extracts isolated from SD exhibit a diverse range of activities in vitro and in vivo, such as anticancer, antioxidant, anti-hepatic injury, anti-inflammatory, and anti-Alzheimer's disease. Furthermore, herbal formulations containing SD have demonstrated significant efficacy in treating various conditions, including chronic gastritis and psoriasis. In summary, this review aims to provide a comprehensive overview of the current research on SD to facilitate its further development and utilization in medicinal applications.
DOI http://doi.org/10.25135/rnp.495.2501.3411 Keywords Scleromitrion diffusum phytochemistry pharmacology clinical application DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.3) Discriminating Metabolites of Curcuma caesia and Kaempferia parviflora through 1H-NMR and GC-MS Analysis for Plant Authentication

The current study aimed to characterize the metabolite fingerprints of the rhizomes of Curcuma caesia Roxb. (KH) and Kaempferia parviflora Wall. ex Baker (HH) in the family Zingiberaceae using a combination of 1H-NMR and GC-MS analyses. There are authentication issues between these two species, wherein both are commonly commercialized as Kunyit Hitam (KH) or Black Turmeric, the local name only for C. caesia. K. parviflora is locally known as Halia Hitam (HH) or Black Ginger. The metabolite profile for each species was analyzed through 1H-NMR data by multivariate data analysis (MVDA) to determine the chemical markers. Orthogonal Partial Least Square-Discriminant Analysis (OPLS-DA) showed clusters of the two species in which the Variable Importance in Projection (VIP) values of more than 1 yielded eleven and four discriminated metabolites belonging to C. caesia (KH) and K. parviflora (HH) respectively. The hexane extracts of the rhizomes of KH and HH were subjected to GC-MS profiling which resulted in 22 and 24 metabolites, respectively and terpenoids, flavonoids, and alkanes were tentatively identified in KH and HH. This study developed characteristic metabolite fingerprinting profiles for each species which distinguish between the rhizomes unambiguously and provide an authenticity assessment of the available products for the patient's benefit
DOI http://doi.org/10.25135/rnp.488.2409.3327 Keywords Curcuma caesia Kaempferia parviflora NMR spectroscopy multivariate analysis authentication marker compounds DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.4) Chemical Constituents of Siegesbeckia orientalis and Their Anti-Proliferative Activity

A new germacrane-type sesquiterpenoid compound (1) and four known compounds (2-5) were isolated from Siegesbeckia orientalis. Chemical structures of these compounds were elucidated using 1D and 2D NMR spectroscopic data, HR-ESIMS, electronic circular dichroism (ECD) and compared with the literature. Their anti-proliferative effects were evaluated on human triple-negative breast cancer (TNBC) cell line MDA-MB-231. The results demonstrated that compound 2 significantly inhibits the proliferation of MDA-MB-231 cells by inducing apoptosis.
DOI http://doi.org/10.25135/rnp.494.2412.3381 Keywords Siegesbeckia orientalis germacrane-type sesquiterpenoids anti-proliferative activity DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.5) Phytochemical Constituents, Antibacterial, Synergistic Antibacterial, Cytotoxic, and Antioxidant Activities of the Essential Oil of Rhizomes of Davallia bullata Wall. ex Hook.

In this study, the phytochemical constituents of Davallia bullata rhizomes essential oil (DBEO) and its biological activities were investigated. According to the GC-FID and GC-MS analysis, the major compounds of DBEO were found to be β-barbatene (17.2%), n-hexadecanoic acid (6.6%), oleic acid (5.7%), nonanal (5.5%), and 1-octen-3-ol (4.9%). For biological activities, DBEO was tested against gram-positive bacteria, B. subtilis and S. aureus, and gram-negative bacteria, E. coli and P. aeruginosa, in order to determine its potential antibacterial activity. The results revealed minimal inhibitory concentration values in the range of 0.16–0.64 mg/mL, while minimum bactericidal concentration values ranged from 0.16 to 1.28 mg/mL. Interestingly, DBEO possessed remarkable synergistic effects when combined with chloramphenicol and streptomycin, with the fractional inhibitory concentration indexes (FICI) varying from 0.13 to 0.50, as determined by the Checkerboard method. Furthermore, DBEO exhibited a moderate level of cytotoxicity against MCF-7, HepG2, HCT-116, and A-549 cells with IC50 values varying from 58.38 ± 0.19 to 94.37 ± 5.09 μg/mL and weak cytotoxicity against non-cancerous HL-7702 cells (177.05 ± 1.34 μg/mL), and the antioxidant capacity of DBEO was evaluated and reported herein.
DOI http://doi.org/10.25135/rnp.498.2501-3404 Keywords Davallia bullata essential oil antibacterial synergistic cytotoxic antioxidant DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.6) Identification of the Main Specialized Metabolites of Ceanothus caeruleus and Cytotoxic Effects of a-nor-Lupane Derivatives†
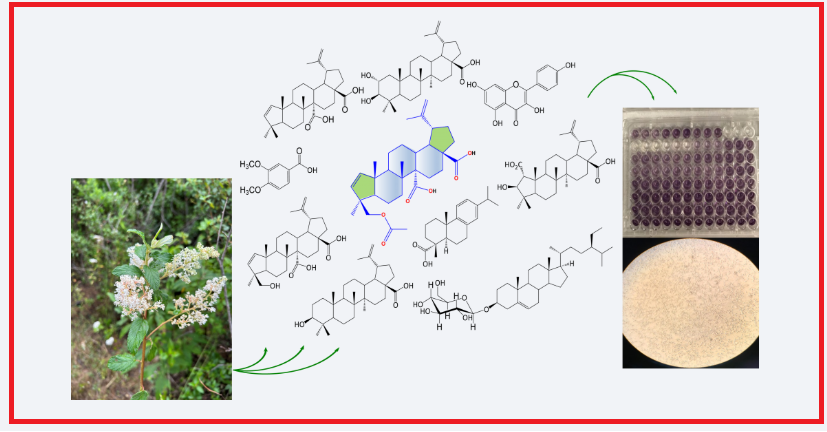
The dichloromethane (DCM) and ethyl acetate (EtOAc) extracts of C. caeruleus yielded nine known compounds, including an A-nor-lupane triterpenoid identified as gouanic acid B (1). Additionally, its acetyl derivative, acetylgouanic acid B (2), is reported here for the first time as a natural product. Furthermore, we tested the bioactivity of natural products 1 and 2 and their dimethyl ester derivatives 3 and 4 against cancer cell lines MCF-7, A549, HeLa, and K562. Among these, compounds 1 (IC50 = 36.4 ± 4.0 μM), 2 (IC50 = 21.6 ± 4.3 μM), and 4 (IC50 = 33.0 ± 2.0 μM) demonstrated moderate to good activity against the K562 cell line while maintaining a satisfactory survival rate in non-cancerous bMEC cells. Notably, the natural triterpenes 1 and 2 and derivative 4 showed remarkable outcomes in cytotoxicity tests due to their specificity against K562 leukemia cells.
DOI http://doi.org/10.25135/rnp.491.2409.3321 Keywords Ceanothus caeruleus triterpenoid bioactive compounds cytotoxicity activity non-cancerous cell line DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.7) A New Dihydrophenanthrene with Cell Viability Enhancing Activities from Spiranthes sinensis

A new dihydrophenanthrene (Spiranthol B), along with four known compounds, were isolated from Spiranthes sinensis. The new structure was determined by various spectroscopic analyses such as 1D, 2D NMR and HRMS techniques. Its stereochemistry was resolved by using the calculated electronic circular dichroism (ECD). Compounds 1-5 showed their capabilities to attenuate palmitic acid (PA)-induced reductions in MIN6 cells viability with the dosages of 3.125 and 6.25 μM.
DOI http://doi.org/10.25135/rnp.492.2410.3345 Keywords Spiranthes sinensis dihydrophenanthrenes cell viability DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.8) 2-Bisabolen-1,10,11,12-tetraol, A New Bisabolane Sesquiterpene from Marine Alga-Sourced Aspergillus taichungensis 299 and Its Antibacterial Activity against Common Pediatric Pathogens
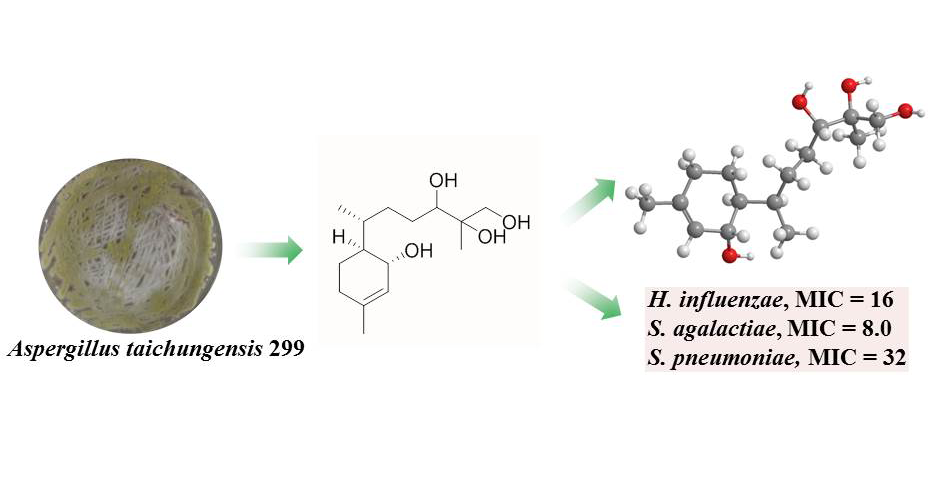
One new bisabolane sesquiterpene, 2-bisabolen-1,10,11,12-tetraol (1), and three known ones, (3R, 6R, 7S, 10S)-1-bisabolen-3,10,11-triol (2), (3R, 6S, 7R)-1,10-bisaboladien-3-ol (3), and nor-bisabolan-1,11-diol (4), were acquired from the extract of Aspergillus taichungensis 299 isolated from the inner tissue of marine red alga Gelidium amansii. Their structures were determined by comprehensive analysis of 1D/2D NMR and HRESIMS data. The antibacterial assay showed that 1 displayed inhibitory activities against the growth of three common pediatric pathogens, Haemophilus influenzae, Streptococcus agalactiae, and Streptococcus pneumoniae, with the MIC values of 16, 8, and 32 μg/mL, respectively.
DOI http://doi.org/10.25135/rnp.496.2501.3394 Keywords Bisabolane sesquiterpene Aspergillus taichungensis structure elucidation antibacterial activity DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.9) Isopimarane Diterpenoids and Pyranones from the Endophytic Fungi Xylaria curta E10
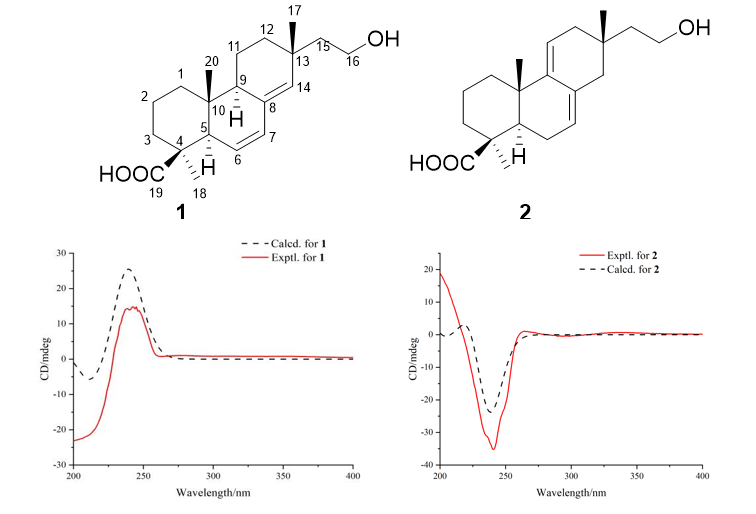
Chemical studies on ethyl acetate extract of solid-state fermentation of Xylaria curta E10 resulted in isolation of two undescribed isopimarane diterpenoids (1 and 2) and two known pyranones (3 and 4). The structures of compounds 1 and 2 were established by spectral data analysis and quantum chemical calculations. The cytotoxic and anti-inflammatory activity of compounds 1 and 2 were evaluated.
DOI http://doi.org/10.25135/rnp.497.2412.3375 Keywords Xylaria curta E10 isopimarane diterpenoids pyranones DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.