Records of Natural Products
Year: 2024 Volume: 18 Issue:1 January-February
1) Plant Products for Musculoskeletal, Respiratory, Circulatory, and Genitourinary Disorders in Eastern and South-Eastern Serbia – Folk Uses Comparison with Official Recommendations

Eastern and south-eastern Serbia is a cultural crossroads between East and West, and due to its economic underdevelopment, the traditional use of medicinal plants remains crucial in healthcare even today. This study aimed to collect and preserve ethnopharmacological knowledge about musculoskeletal, respiratory, circulatory, and genitourinary disorders, which are common in the local population. Information was collected using semi-structured anonymous ethnobotanical interviews with location informants. According to respondents, monographs of official international authorities (European Pharmacopoeia, ESCOP, WHO, EMA, and PDR) have been reviewed to confirm the traditional use of medicinal plants. Out of a total of 161 respondents, 58 (36%) declared that they use plants to treat musculoskeletal diseases, 147 (91.3%) to treat respiratory diseases, 113 (70.19%) to treat circulatory diseases, and 25 (15.53%) to treat genitourinary diseases. Among the plants that are traditionally used for the treatment of diseases of these organ systems, the following are highlighted for future research: Verbascum phlomoides, Inula helenium, and Rosmarinus officinalis for musculoskeletal; Ocimum basilicum, Robinia pseudoacacia and Primula vulgaris for respiratory; Urtica dioica, Allium ursinum, and Rosa canina for circulatory and Apium graveolens, Zea mays and Calendula officinalis for treatment of genitourinary system.
DOI http://doi.org/10.25135/rnp.428.2308.2861 Keywords Ethnopharmacological study Balkan region musculoskeletal respiratory circulatory genitourinary disorders DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.2) Panacis majoris Rhizoma: A Comprehensive Review of Phytochemistry, Pharmacology and Clinical Applications

Panacis majoris Rhizoma (PMR) is a traditional Chinese medicine with tonic, hemostatic, and pain-relieving properties, classified under the genus Panax in the family Araliaceae. With a lengthy history of folk usage, PMR holds considerable potential for further development and exploration. Modern research indicates that saponins are the main active constituents. To date, a total of 122 compounds have been isolated from PMR, with the vast majority being triterpenoid saponins. In addition, there are small amounts of flavonoids, aromatic hydrocarbons, alkynes, and volatile oils. Pharmacological studies have demonstrated that compounds and extracts from PMR exhibit notable activities, including hepatoprotective, anti-cancer, cardioprotective, anti-ischemic brain injury, antioxidant, anti-inflammatory, analgesic, and anticoagulant effects. These compounds, serving as precursor molecules for drug development, are of significant value. This article provides a comprehensive review of PMR’s botany, phytochemistry, pharmacology, and clinical applications, offering valuable insights for its subsequent development and resource utilization.
DOI http://doi.org/10.25135/rnp.425.2310.2917 Keywords Panacis majoris phytochemistry pharmacology clinical applications DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.3) Anthraquinones and Macrocyclic Lactones from Endophytic Fungus Penicillium roseopurpureum and Their Bioactivities

Endophytic fungi colonize the internal and distinct tissues of the host plants. In recent years, there has been growing interest in endophytic fungi as valuable sources for drug discovery based on their rich metabolic profiles consisting of novel and bioactive compounds. Accordingly, our preliminary study demonstrated that an endophyte, namely Penicillium roseopurpureum isolated from Astragalus angustifolius, had high chemical diversity with an antiproliferative effect. Herein, fermentation of P. roseopurpureum resulted in the production of five new anthraquinone-type compounds (2, 4, 6, 7, 8) together with several known compounds [11-methoxycurvularin (1: epimeric mixture of 1a and 1b), carviolin (3), 11-hydroxycurvularin (5: diastereoisomeric mixture of 5a and 5b) and 1-O-methylemodin (9)]. The structures of the new compounds were established by NMR spectroscopic and HRMS analysis. Cytotoxicity studies demonstrated that none of the compounds except for 1 and 5 had antiproliferative activity against prostate cancer cell lines. Interestingly, 1 was found as cytotoxic, whereas 5 exhibited cytostatic properties. Also, 7-AAD/Annexin V staining supported these results by showing that 1 caused cellular death, while 5 did not show any increase in dead cell content in comparison to the control. Lastly, cell cycle analysis showed that compounds had distinctive cell cycle arrest patterns.
DOI http://doi.org/10.25135/rnp.426.2309.2896 Keywords Endophytic Fungi natural Products anthraquinones macrocyclic Lactones cytotoxicity cell cycle arrest DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.
4) Pterosterone 20,22-Acetonide, a New Ecdysteroid and Other Constituents from Acrostichum aureum L.

Chemical investigation of the EtOAc extract from the aerial parts of Acrostichum aureum distributed along coastlines of Vietnam lead to the isolation of a new ecdysteroid, named pterosterone 20,22-acetonide (1), along with twelve known compounds (2-13). Their structures were elucidated by spectroscopic methods including 1D, 2D NMR and HR-ESI-MS analysis as well as comparison with the results of previous studies. Five ecdysteroids (1-5) were evaluated for their cytotoxic activity against the LU-1, MCF7 and HepG2 human cancer cell lines using the SRB assay. Ecdysteroids 1 and 2 having a 20,22-dimethyl acetal group showed cytotoxicity against all tested cell lines with IC50 values in the range 51.59 to 60.14 μM. The other compounds were considered as inactive in this test.
DOI http://doi.org/10.25135/rnp.431.2311.2971 Keywords Acrostichum aureum Pteridaceae ecdysteroid flavonoid spectroscopic analyses cytotoxicity DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.5) Chemical Profiles and Bioactivities of the Essential Oils from Four Lauraceae Plants for Controlling Tribolium castaneum Herbst

Essential oils (EOs) from Lauraceae plants have been extensively investigated in the control of stored-product insects. In this work, four Lauraceae species namely Lindera communis Hemsl., Phoebe neurantha (Hemsl.) Gamble, Litsea rotundifolia Hemsl. var. oblongifolia (Nees) Allen and Litsea variabilis Hemsl. var. oblonga Lec. were collected for extracting EOs by hydro-distillation and their chemical compositions were comparatively analyzed by GC/MS and GC/FID. Furthermore, contact toxicity and repellency of these EOs were evaluated against Tribolium castaneum Herbst, a universal model insect used in fundamental and applied research. Results indicated that EOs from Lin. communis, P. neurantha, Lit. rotundifolia and Lit. variabilis were mainly composed of sesquiterpenoids including (E)-β-famesene, cis-α-bisabolene, α-selinene, eremophilene, β-selinene, etc. In bioassays, all the EOs at maximum testing concentration of 78.63 nL/cm2 were significantly repellent to T. castaneum adults at 2 and 4 h post-exposure, which were comparable to the positive control DEET. Among them, EOs from P. neurantha, Lit. rotundifolia, Lin. communis P and Q also had contact toxicity with LD50 values of 14.52, 17.58, 23.82 and 86.63 µg/adult respectively. It suggests that the four species of Lauraceae have promising potential to be developed into botanical repellents and contact toxicants against stored-product insects.
DOI http://doi.org/10.25135/rnp.432.2309.2905 Keywords Lindera communis Phoebe neurantha Litsea rotundifolia var. oblongifolia Litsea variabilis var. oblonga Biopesticides Stored-product insects DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.6) Allium hookeri Enhances Muscular Endurance of Mice by Increasing Muscle Cross-Sectional Area

Despite the known physiological effects of Allium hookeri Thwaites (A. hookeri) on metabolism, the effects of ingestion of the extract on hematological parameters and exercise capacity are largely unknown. We aimed to investigate the hypothesis that water extracts of A. hookeri root (WEAH) would improve hematological parameters and promote exercise capacity. The intake of WEAH-containing feed significantly reduced the weight of white adipose tissue and the amount of total cholesterol in the blood, but it did not affect the body weight of mice. WEAH intake enhanced the muscular endurance of mice in treadmill endurance tests. The cross-sectional areas of muscle fibers in the gastrocnemius muscle of the WEAH-fed mice were larger than those in the control mice. WEAH promoted myoblast myogenesis by increasing the expression of the myogenic protein myosin heavy chain 3 and myotube formation. Taken together, our results suggest that A. hookeri may be valuable in the development of preventive and therapeutic medicines for sarcopenia as well as in providing basic knowledge of muscular functions.
DOI http://doi.org/10.25135/rnp.433.2310.1929 Keywords Allium hookeri muscular endurance muscle cross-sectional area myosin heavy chain 3 myoblast DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.7) A New Ascochlorin Glycoside from Brittlestar-derived Fungus Acremonium sp. and Its Biological Activities

One new ascochlorin glycoside (1) and 8 known ascochlorin derivatives (2–9), and ilicicolin H (10) were purified from brittlestar derived fungus Acremonium sp. GXIMD02024. Their structural elucidations were carried out by analyses of 1D and 2D NMR, high resolution mass spectrometry, and CD spectroscopic data and comparison of those with literature data. Compound 1 was a rare fungal polyketide-sesquiterpenoid possessing sugar moiety. The antibacterial and cytotoxicity activities of compounds 1–10 have been performed in vitro. Compounds 1–3, 7, 8, and 10 were measured for their inhibitory effect on a-glucosidase. Compound 1 exhibited poor activity or was inactive, but the detail analysis indicated sugar moiety in 1 maybe play an important role in effect on biological activities.
DOI http://doi.org/10.25135/rnp.438.12310.2940 Keywords Ascochlorin glycoside Acremonium sp α-glucosidase inhibitory activity marine fungus DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.8) Protective Effects of Origanum onites and Its Components on Lead-Nitrate Induced Genotoxicity in Root Cells of Allium cepa L.

This study investigates the protective effects of components (Thymol; Thy, Carvacrol; Car, Linalool; Lin, and α-Pinene; AP) and essential oil of Origanum onites L. (O. onites-EO), against lead nitrate-induced cytotoxicity and genotoxicity in Allium cepa L. (A. cepa) root tip cells. These components obtained from O. onites were characterized by gas chromatography (GC). A. cepa bulbs were exposed to 6.25-12.5 mg.L-1 concentrations of the O. onites-EO/components of O. onites-EO for analyses of induction of cytogenetic damage. Then, these bulbs were exposed to 10 mg.L-1 concentrations of lead nitrate for analyses of the protective effects of O. onites and its components. Mitotic abnormalities were evaluated for genotoxicity, and mitotic index (MI) for cytotoxicity. As a result of this study, lead nitrate increased the total chromosomal abnormality amount in A. cepa, indicating genotoxicity. MI was decreased with lead nitrate. However, this effect was significantly improved by components of O. onites-EO. This effect was shown with the decrease in the number of chromosomal abnormalities and increase in MI rates in lead nitrate-induced root cells after exposure to the components of O. onites-EO. The protective effect of O. onites-EO components against the damage caused by lead nitrate in cells can be listed as α-Pinene > Thymol > Carvacrol > Linalool. Among all essential oil components tested, α-Pinene was determined to have the strongest protective effect. Furthermore, the protective effect of the essential oil, which contains all the components, could not be determined. It has been observed that the components of essential oil have different effects, and it can be said that these components suppress the effects of each other in the mixture where they are found together. In conclusion, this study shows that the components of O. onites-EO have a protective effect on lead nitrate-induced A. cepa root cells.
DOI http://doi.org/10.25135/rnp.440.2311.2990 Keywords Anti-genotoxicity essential oil heavy metal toxicity lead-nitrate Origanum onites DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.9) A New Aporphine Alkaloid from Litsea glutinosa to Attenuate Palmitate Induced Viability in MIN6 Cells

A new alkaloid called Litsine E (1) and three known aporphine alkaloids (2-4) were isolated from Litsea glutinosa. The structure of the new compound was established using spectroscopic techniques such as HMBC, HSQC, COSY, NOESY, and HRESIMS. Electronic circular dichroism (ECD) calculations were used to estimate its absolute configuration. Subsequently, the newly identified compound was evaluated for its potential to attenuate palmitate-induced viability, demonstrating a significant increase in viability in MIN6 cells.
DOI http://doi.org/10.25135/rnp.427.2308.2875 Keywords Litsea glutinosa aporphine alkaloids palmitate-induced viability DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.10) Two Polyoxygenated Bipyrrole Alkaloids from Speranskia tuberculata

Three polyoxygenated bipyrrole alkaloids were isolated from the aerial of Speranskia tuberculata, including two new compounds, speranberculatines B (1) and C (2), along with a known compound, speranskatin A (3). Their structures were identified via NMR-spectroscopic and MS analyses. None of them showed activity in inhibiting the production of NO in LPS-induced RAW264.7 cells.
DOI http://doi.org/10.25135/rnp.434.2311.2972 Keywords Speranskia tuberculata bipyrrole alkaloid structure elucidation DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.11) Chemical Components and Their α-Glucosidase Inhibitory Activity from the Leaves of Ficus carica Linn.
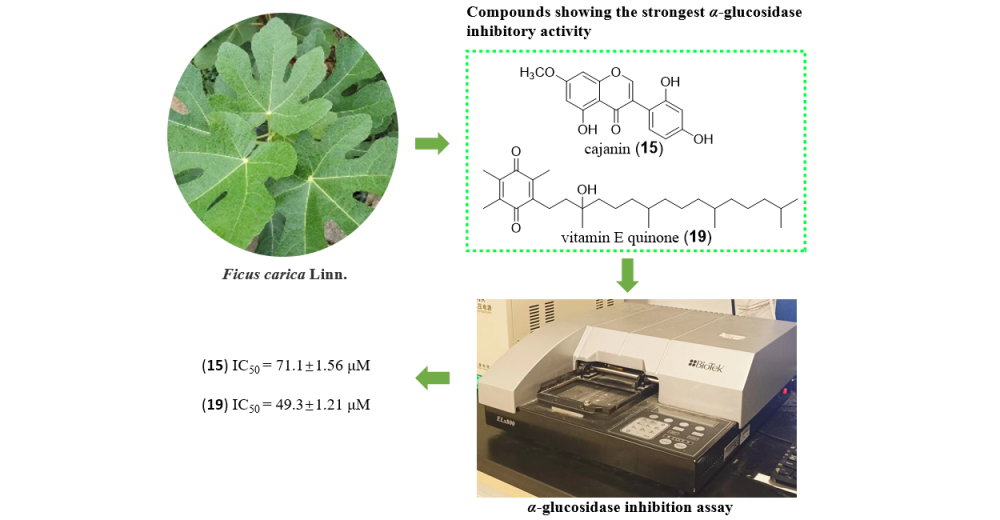
Nineteen compounds were purified from leaves of Ficus carica Linn. using various separation techniques such as recrystallization, column chromatography filled with silica gel, Sephadex LH-20, and MCI, and semi-preparative HPLC. By analysis of NMR, MS, and data comparison with those reported from literatures, their structures were identified as umbelliferone (1), psoralen (2), furopinnarin (3), 6,7-furano-hydrocoumaric acid (4), (E)-3-[5-(6-hydroxy) benzofuranyl] propenoic acid (5), (E)-3-(6-hydroxy-4-methoxy-5-benzofuranyl) propenoic acid (6), nodakenetin (7), oxypeucedanin hydrate (8), dihydrofurocoumarin (9), (E)-4-hydroxy-3,3,5-trimethy1-4-(3-oxobu-1-en-1-yl)-cyclohexan-1-one (10), dehydrovomifoliol (11), 4,5-dihydroblumenol A (12), blumenol A (13), 5-hydroxy-4′,7-dimethoxyisoflavone (14), cajanin (15), loliolide (16), indole-3-carboxaldehyde (17), 1H-indole-3-carboxylic acid (18), vitamin E quinone (19). Their α-glucosidase inhibitory activities were evaluated by IC50 values. Compounds 15 and 19 showed obvious α-glucosidase inhibitory activity with IC50 of 71.1±1.56 µM and 49.3 µM±1.21, respectively, and by the kinetics of enzymatic reaction, the inhibition type by which compound 15 acting against α-glucosidase was inferred as anticompetitive. Moreover, preliminary structure-activity relationship was recapticulated from the tested compounds.
DOI http://doi.org/10.25135/rnp.430.2320.2935 Keywords Ficus carica Linn. coumarin α-glucosidase inhibitor enzyme kinetics DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.12) A New Phenolic Bisabolane Sesquiterpenoid from the Fungus Aspergillus sp. Hvtc-2021zx1

The strain Aspergillus sp. Hvtc-2021zx1 was isolated and cultivated in solid-state fermentation using rice medium. The medium was extracted with ethyl acetate (EtOAc) to give an extract, which was separated by various chromatographic techniques to give five compounds including the new bisabolane 1. The structures were determined by 1H NMR, 13C NMR, 2DNMR and ESIMS data. It should be noted that an O-methyl group at C-7 as in compound 1 was rarely found in the phenolic bisabolane skeleton. The four known compounds 2-5 were identified to be 11,12-dihydroxysydonicacid (2), sydowic acid (3), cyclo(D-phenylalanyl-L-leucyl) (4), and neoechinulin A (5), respectively. The NMR data of 5 was reported for the first time in DMSO-d6. Compound 5 was active against S. aureus with an MIC value of 64 ug/mL
DOI http://doi.org/10.25135/rnp.429.2310.2939 Keywords Phenolic bisabolanes Aspergillus sp. Hvtc-2021zx1 DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.