Organic Communications
Year: 2023 Volume: 16 Issue:2 April-June
1) Synthesis of heterocyclic compounds from camphor
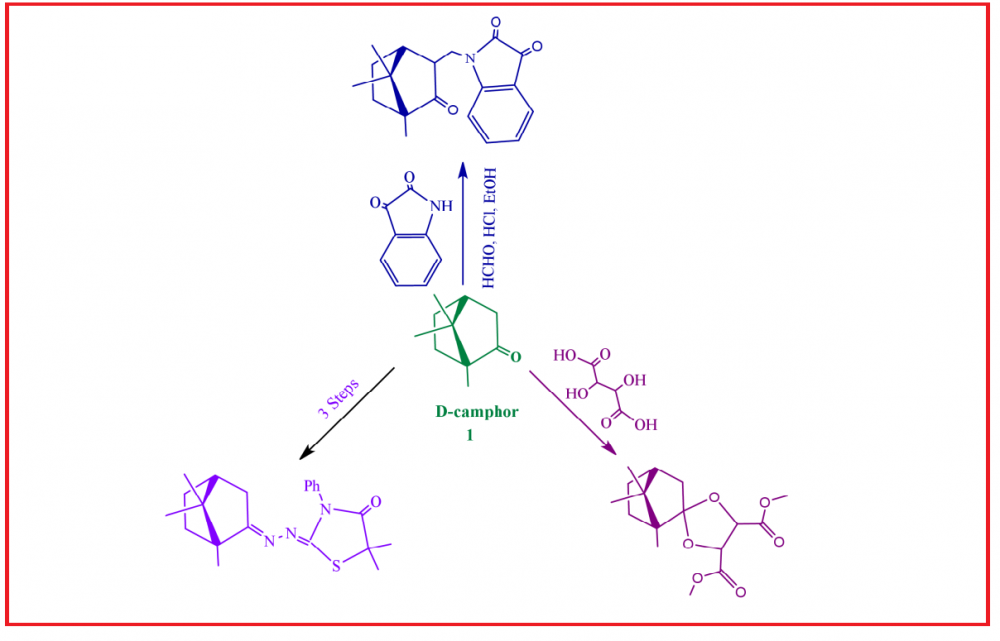
In this review, we demonstrated the synthesis of isatin derivative 3 according to the Mannich reaction. Moreover, the synthesis of substituted enamines 6a-b using urea catalysts were studied. Additionally, the synthesis of azepanes, piperidines, pyrrolidines, pyrazole, pyridine and pyrimidine derivatives were reported. Furthermore, the synthesis of triazolium salts (34), enamines derivatives 39 and 40, tetrapyrazinoporphyrazine magnesium complex (43), ligands 49 and 50, optically active α-amino acids 62a, lactam derivative 67, and its isomer α-camphidone (68), camphor dimethyl DL‑tartrate (Ct diester) (70), and thiazole derivatives from camphor monoterpenes were realized. The biological activities of many compounds were studied toward human cancer cell lines, influenza virus, Streptococcus pneumoniae, Klebsiella pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, Methicillin-Resistant Staphylococcus aureus (MRSA), Escherichia coli, Bacillus cereus, Bacillus subtilis and vaccinia virus, showing interesting results.
DOI http://doi.org/10.25135/acg.oc.150.2303.2741 Keywords Camphor pyrazole dimethyl DL tartrate thiazole biological activity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.2) Synthesis and biological studies of novel hydroxyacetophenone-tetrazole hybrids
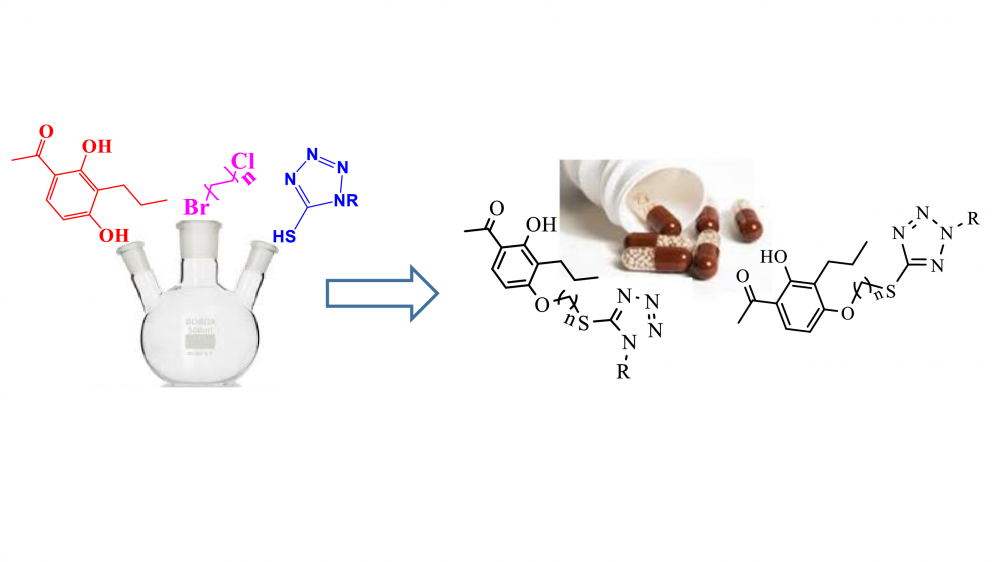
A series of novel hybrid compounds was synthesized where 2′-hydroxyacetophenone and N-alkylated thiotetrazole were coupled via methylene spacers of different lengths. The structures of the compounds were characterized by spectroscopic techniques, including the 1H- and 13C-NMR, FT-IR, and HRMS methods. The antimicrobial activities of the title compounds were evaluated against clinical isolates of Gram-positive and negative bacterial and fungal species. All compounds showed a broad spectrum of antimicrobial activity and inhibited all bacteria and fungi that tested with MIC values ranging from 4 to 128 µg/ml. 4a and 5d were the most active antibacterial members.
DOI http://doi.org/10.25135/acg.oc.148.2302.2696 Keywords Hydroxyacetophenone tetrazole hybrid antimicrobial activity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.3) Green synthesis of 3,4-disubstituted isoxazol-5(4H)-one using Gluconic acid aqueous solution as an efficient recyclable medium.
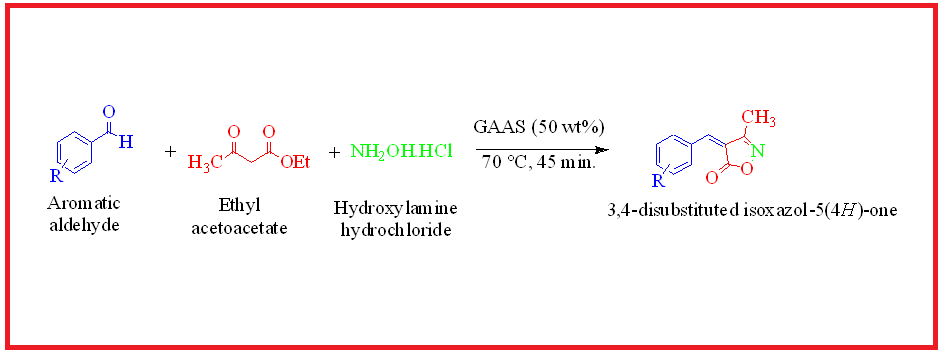
Green synthesis of 3,4-disubstituted isoxazol-5(4H)-ones has been achieved via Knoevenagel condensation using three-component coupling reaction of aromatic aldehyde, ethyl acetoacetate and hydroxylamine hydrochloride in Gluconic acid aqueous solution (50 wt % GAAS). In this methodology, Gluconic acid aqueous solution used as reaction medium as well as catalyst and it can be recycled and reused several times without loss of its a significant efficacy. All compounds have been confirmed by 1H NMR, 13C NMR, IR spectroscopy and mass spectrometry.
DOI http://doi.org/10.25135/acg.oc.151.2304.2762 Keywords Green synthesis gluconic acid aqueous solution bio-based green solvent Knoevenagel condensation 3,4-disubstituted isoxazol-5(4H)-ones. DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.4) Methoxyphenanthrenes: synthesis and structure analysis
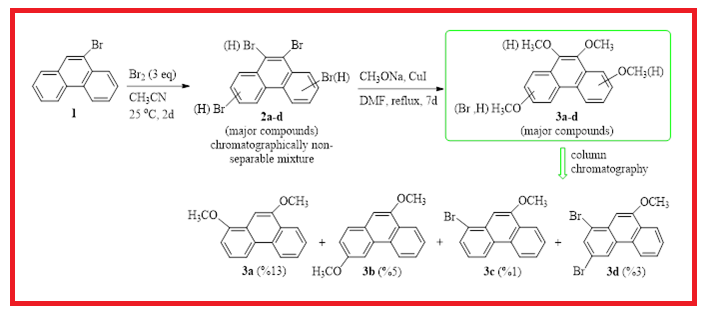
The phenanthrene bromide product mixture obtained from bromination of 9-bromophenanthrene 1, which could not be separated chromatographically, was converted to chromatographically separable methoxy products (3a-d) with CuI catalyzed methoxylation reaction. The resulting products were purified by chromatographic method and detailed structure analysis of the products was done by NMR spectroscopy. Two-dimensional NMR spectroscopy methods can be used successfully to elucidate the structures of methoxyphenanthrene derivatives. With the combination of APT, DEPT 90, HETCOR, HMBC, COSY and NOESY spectral data, the position of the substituents was easily determined. In particular, the HMBC and NOESY spectra provide critical information about the positions of the bounded groups. Further bromination reactions of 1,9-dimethoxide 3a selectively gave brominated compound 4.
5) Efficient and regioselective acetylation of benzene derivatives with Ac2O in the presence of mercurytetrathiocyanatocobaltate (II)
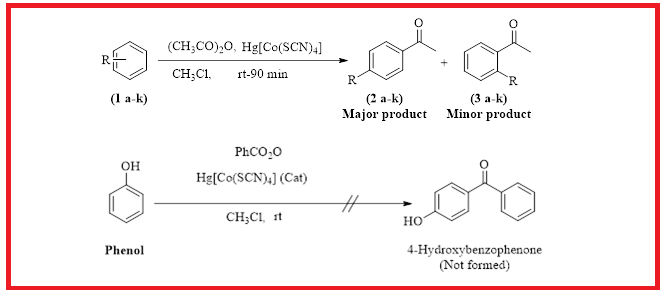
Based on Friedel Crafts acylation, an efficient and facile method for the chemo-selective as well as region-selective acylation of substituted benzene derivatives has been developed by using Hg[Co(SCN)4] as catalyst. The reaction is highly chemo-selective as well as region-selective as only acetophenones were obtained, whereas the benzophenones are not formed. The present method is very efficient with simple work up gives the products in good to excellent yield. The reaction is carried out using CHCl3 as solvent at room temperature. It is anticipated that the present newly developed method will open a gateway for the chemist to prepare the chemo as well as region-selective acetophenones.
DOI http://doi.org/10.25135/acg.oc.153.2304.2760 Keywords Regioselective Hg[Co(SCN)4] aromatic ketones DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.6) An efficient zinc acetate dihydrate-catalyzed green protocol for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones
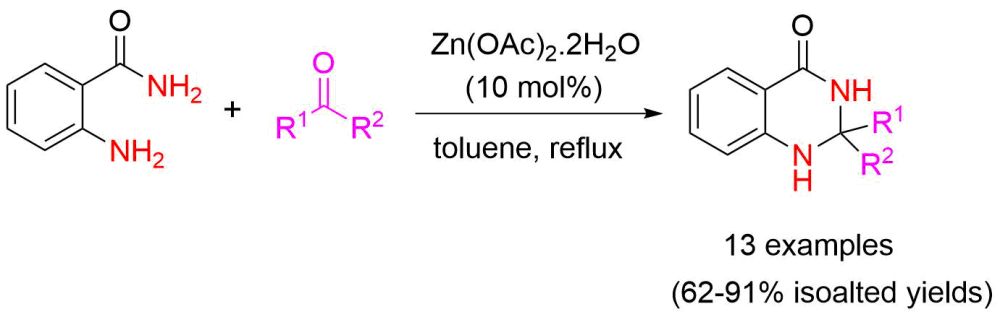
By condensation from substituted carbonyl compounds and anthranilamide under toluene reflux conditions, a wide range of 2,3-dihydroquinazolin-4(1H)-ones were produced in fair to good yields with the use of a Lewis acid catalyst Zn(OAc)2•2H2O (10 mol%), which is inexpensive, accessible, and environmentally friendly. All the synthesized compounds were properly described using melting point, IR, NMR, and mass spectral studies, and the findings were compared with information from the earlier literature. The new method has a number of advantages over the traditional methods for the synthesis of divergent 2,3-dihydroquinazolin-4(1H)-ones, including a higher product conversion, a wide substrate range, and the absence of undesirable side products. Aliphatic, heteroaromatic and aromatic carbonyl compounds were well tolerated under the optimized reaction conditions.
DOI http://doi.org/10.25135/acg.oc.149.2303.2742 Keywords Quinazoline 2,3-dihydroquinazolin-4(1H)-ones catalysis zinc acetate dihydrate (Zn(OAc)2.2H2O) cyclization heterocycles DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.