Records of Natural Products
Year: 2023 Volume: 17 Issue:1 January-February
1) Editorial
2) Ethnobotanical Records of Medicinal Plants of Turkey Effective on Stress Management Complied with the Literature Survey in Their Chemical Content and Activities
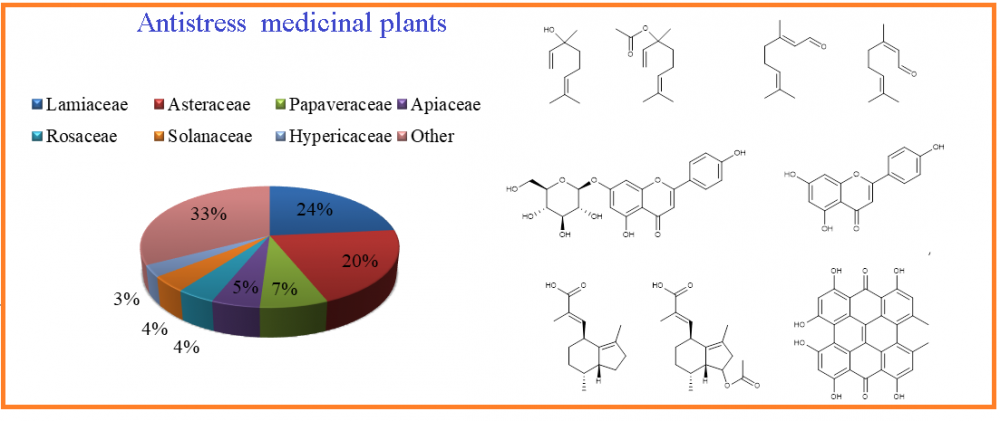
The increase of challenges in people's lives, daily problems as well as traumatic events could lead them to experience stress. Because of the side effects of current drugs, the recent medications are not sufficient to cure stress-related diseases; new approaches are needed in order to find more effective medications with fewer side effects. Ethnobotanical and ethnomedical research is increasingly recognized as a viable source of data and plausible pharmacological action of many plants. The review presents ethnobotanical information of the plants which have been used against stress-related diseases among local people of Turkey. In addition, a survey of the current literature on the topic aims to find new natural resources that will contribute to the development of drugs and bring them to the literature by scanning the scientific articles on the isolation and structure determination of the secondary metabolites of these medicinal plants, which have been already in use among the public for stress-related disorders for centuries. This research is not only the first step in the research of promising new compounds against stress but it is also a presentation of data on medicinal plants of Turkey: Their medicinal parts, method of preparation, usage patterns and, if recorded, their dosages.
DOI http://doi.org/10.25135/rnp.324.2203.2372 Keywords Stress herbal drugs ethnobotanical DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.3) A Novel Cyclohexane Carboxylic Acid Derivative from Black Turtle Bean (Phaseolus vulgaris L.)

Abstract: Black turtle bean is a variety of Phaseolus vulgaris L. in the legume family Fabaceae and is consumed as food worldwide. A targeted phytochemical investigation of the ethanolic extract of its seeds, focusing on constituents other than the lipophilic metabolites, resulted in the isolation and characterization of five compounds (1-5). Compound 1 (phasvulic acid), a previously undescribed cyclohexane carboxylic acid derivative, was characterized as (Z)-3-hydroxy-2-(5-hydroxypent-2-en-1-yl)cyclohexane-1-carboxylic acid based on spectral data including 1D and 2D NMR (1H, 13C, COSY, HSQC, and HMBC) and high-resolution electrospray ionization mass spectrometry (HR-ESI-MS). Other compounds were formerly described as dihydrophaseic acid (2), uridine (3), stigmasterol-3-O-β-D glucopyranoside (4), and β-sitosterol-3-O-β-D-glucopyranoside (5).
DOI http://doi.org/10.25135/rnp.325.2202.2361 Keywords Black turtle bean Phaseolus vulgaris phasvulic acid legume Fabaceae DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.4) Secondary Metabolites with Antioxidant and Mushroom Tyrosinase Inhibitory Activities from Ajuga nipponensis
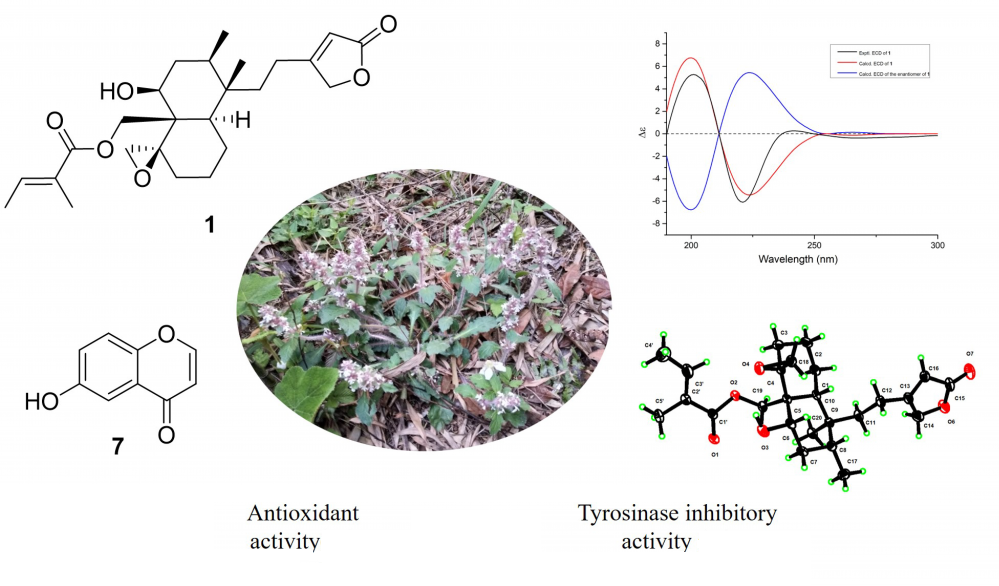
One neo-clerodane diterpenoid (1), two abietane diterpenoids (2 and 3), three flavonoids (3–6), one C6-substituted chromone derivative (7), one phenylpropanoid (8), as well as one chain fatty acid (9) were isolated from the whole plants of Ajuga nipponensis. Their structures were established by detailed analysis of the HRESIMS, 1D and 2D NMR, UV, and IR. The absolute configuration of compound 1 was firstly determined by ECD calculation and single crystal diffraction. The NMR data of compound 9 are reported for the first time. Compounds 4, 7–9 were isolated from this medicinal plant for the first time, and 4, 9 were first discovered from natural products. The isolates 1–7 were tested for their antioxidant and mushroom tyrosinase inhibitory activities. Most of them showed moderate antioxidant activities. Compounds 1, 4, 5, and 7 exhibited obvious antioxidant activities at 50 μM, with % inhibition values of 32.37±0.75%, 25.30±0.66%, 28.76±0.64%, 31.06±0.46%, respectively, with L-ascorbic acid used as the positive control (71.29±0.34%). Compound 7 exhibited significant inhibitory activities against mushroom tyrosinase (%inhibition values of 16.94±1.28%), with arbutin used as the positive control (29.9±0.99%).
DOI http://doi.org/10.25135/rnp.321.2201.2330 Keywords Ajuga L Ajuga nipponensis diterpenoid flavonoid antioxidation tyrosinase inhibitory activity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.
5) First 6, 7-Seco-Clerodane Furan Diterpenoid from Croton morifolius
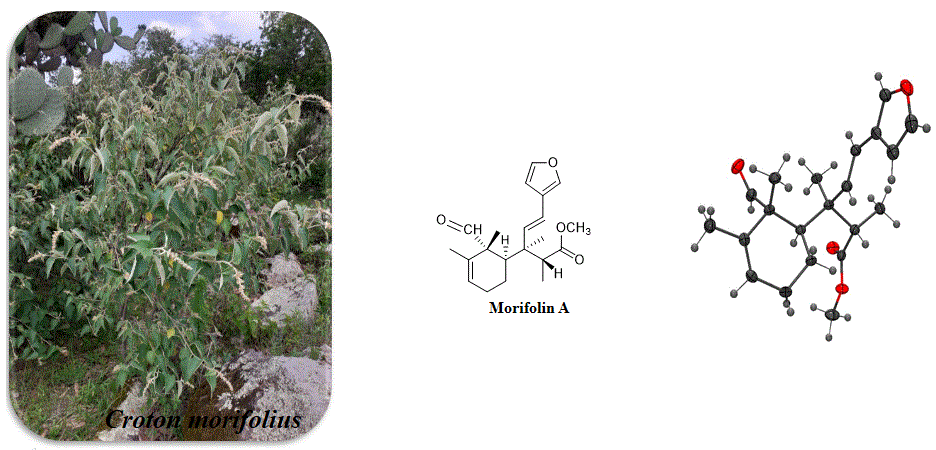
A novel 6,7-seco-clerodane furan-diterpenoid, named as Morifolin A, was obtained from Croton morifolius. The molecular structure of Morifolin A was determined by 1D/2D NMR spectroscopy, mass spectrometry and single-crystal X-ray diffraction analysis (Cu Kα). Morifolin A contains one furan ring bridged to a cyclohexene ring via an alkenyl chain and its crystal packing is displaying intermolecular interactions as C−H⋯O.
DOI http://doi.org/10.25135/rnp.332.2204-2422 Keywords Croton morifolius 6,7 seco-clerodane furan-diterpenoid Morifolin A absolute configuration X-ray diffraction DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.6) The Essential Oil Compositions of Teucrium spp. Belonging to the Section Polium Schreb. (Lamiaceae) Growing in Cyprus
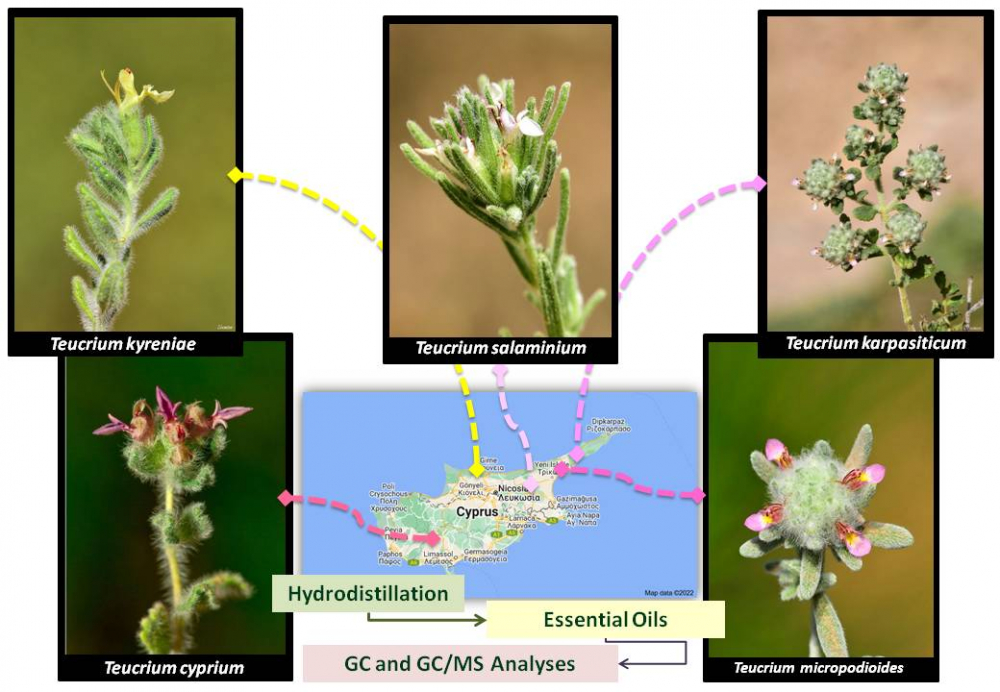
The genus Teucrium L., belonging to the family Lamiaceae, is divided in ten sections including Polium (Mill.) Schreb. In Cyprus, this section is represented by five endemic species: T. micropodioides, T. cyprium, T. karpasiticum, T. kyreniae and T. salaminium. Essential oils of the aerial parts of these species were separately obtained by hydrodistillation using a Clevenger-type apparatus. Chemical compositions of the obtained oils were analyzed by GC and GC/MS, simultaneously. The essential oil yields ranged between 0.03-0.75 (v/w). The major components of the essential oil of T. kyreniae were β-caryophyllene (21.2%), (Ε)-nerolidol (10.9%), carvone (8.8%) and α-humulene (7.9%). T. salaminium essential oil was dominated by β-caryophyllene (15.2%), caryophyllene oxide (13.9%), germacrene D (11.6%) and bicyclogermacrene (10.0%). Major components of the oil of T. cyprium were β-pinene (35.4%), α-pinene (10.6%) and spathulenol (8.4%). Major compounds of the oils of T. micropodioides samples, collected from two different locations, were (1) limonene (47.0%), β-pinene (16.0%) and β-caryophyllene (6.9%), (2) δ-cadinene (11.2%), 1-epi-cubenol (7.8%), cubebol (4.7%), cubenol (4.1%), respectively. Main constituents of the essential oil of T. karpasiticum were limonene (43.9%), β-pinene (16.0%), α-bisabolol oxide A (7.8%), β-caryophyllene (5.5%) and α-pinene (4.6%). The section Polium, is considered a complex and the diversity of oil compositions from the species belonging to this section provided additional proof to this consideration.
DOI http://doi.org/10.25135/rnp.327.2203.2379 Keywords Lamiaceae Section Polium Teucrium spp. Cyprus Endemic Essential oil DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.7) In Silico Study of Natural Xanthones as Potential Inhibitors of Alpha-Glucosidase and Alpha-Amylase
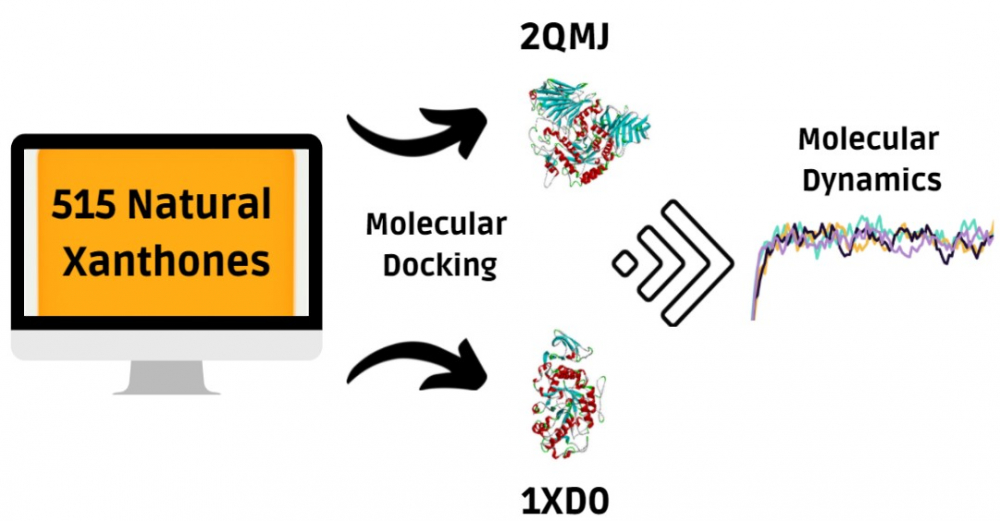
Type 2 diabetes mellitus is a disease caused by insulin resistance. Many types of oral medications exist, but the effectiveness and side effects differ from patient to patient, so alternative drugs are still required. One compound group receiving much scientific interest regarding its antidiabetic potential is xanthones as potential alpha-glucosidase and alpha-amylase inhibitors. This study performed molecular docking simulations on all 515 natural xanthones with alpha-glucosidase and alpha-amylase as the protein targets. We found 31 unique ligands that comply, and the three best ligands per protein target were filtered based on how many active site residues the ligands interacted with the targets. The three best alpha-glucosidase inhibitors are 3,4,5,8-tetrahydroxy-1,2-diisoprenylxanthone, Polygalaxanthone V, and Polygalaxanthone VII. As for alpha-amylase, we found 1-O-primeverosyl-3,8-dihydroxy-5-methoxyxanthone, Garcimangosone C, and Mangostinone as the best inhibitors. The six chosen and standard ligands underwent 2 ns molecular dynamics simulations. Both standard ligands had the highest interaction energies, followed by complexes with glycosylated xanthones, and prenylated xanthones. We also found that the prenylated xanthones could retain their initial protein-ligand interactions. Therefore, this is the first study that revealed prenylated xanthones have a good potential as anti-type 2 diabetes mellitus agents among other xanthones groups through in silico method.
DOI http://doi.org/10.25135/rnp.337.2203.2391 Keywords Molecular docking molecular dynamics xanthone alpha-amylase inhibitor alpha-glucosidase inhibitor DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.8) Novel Acyclic Diterpene from Bornean Local Red Chilli Pepper Capsicum frutescens L.
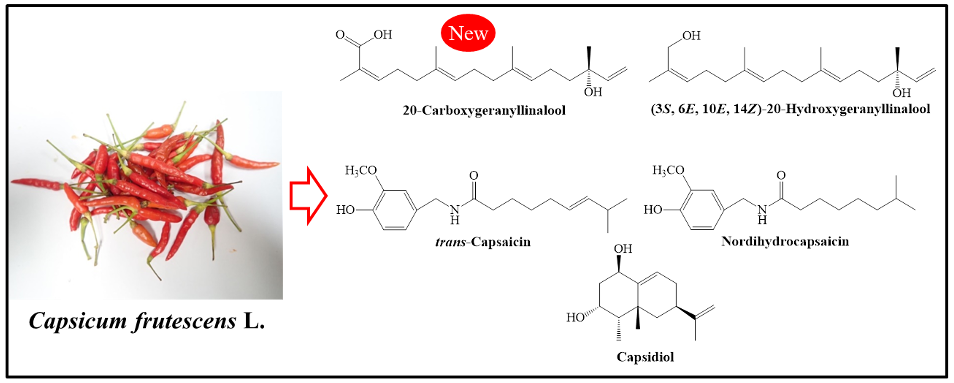
An unknown acyclic diterpene (3S, 6E, 10E, 14Z)-3-hydroxy-3,7,11,15-tetramethyl-1,6,10,14-hexadecatetraene acid (1) along with four known secondary metabolites (3S, 6E, 10E, 14Z)-20-hydroxygeranyllinalool (2), trans-capsaicin (3), nordihydrocapsaicin (4) and capsidiol (5) were isolated from the Bornean red chilli pepper Capsicum frutescens L. The structures of the secondary metabolites were determined based on spectroscopic data analysis such as NMR, HRESIMS, and IR data. The new compound 1 is a carboxylic acid precursor that would condensate with vanillylamine in the phenylpropanoid pathway in the biosynthesis of capsaicinoids. Discovery of this compound is an important milestone in our understanding of the capsaicinoids biosynthesis.
DOI http://doi.org/10.25135/rnp.339.2204.2443 Keywords Red Chilli Capsicum frutescens L. Bornean acyclic diterpene DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.9) Two New Disaccharide Glycosides from the Root Cortex of Paeonia ostii
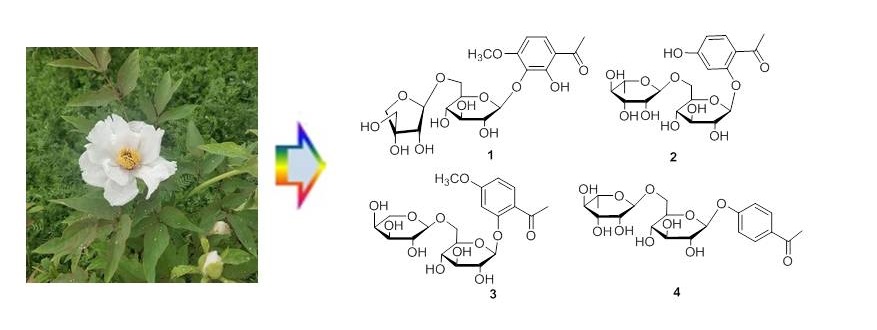
Two new glycosides 2-hydroxy-4-methoxy-acetophenone-3-O-[β-D-apiofuranosyl (1→6)-β-D-glucoside (1) and 4-hydroxy-2-O-β-rutinosyl acetophenone (2), along with two known compounds, paeonolide (3), involcranoside B (4) were isolated from the roots of Paeonia ostii. In addition, compound 4 was isolated from this genus for the first time. Their structures were established on the basis of spectral and chemical evidence. All the compounds showed inactive nitric oxide (NO) inhibitory effects.
DOI http://doi.org/10.25135/rnp.342.2206.2475 Keywords Paeonia ostii disaccharide glycosides water-soluble extraction anti-inflammatory DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.10) Chemical Constituents from the Whole Plant of Pachysandra terminalis
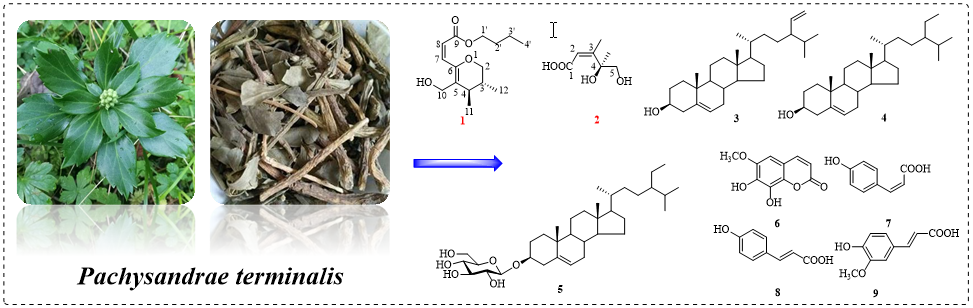
Two new compounds, butyl(Z)-3-((3R,4R)-5-(hydroxymethyl)-3,4-dimethyl-3,4-dihydro- 2H-pyran-6-yl)acrylate (1) and (2Z,4S)-4,5-dihydroxy-3,4-dimethylpent-2-enoic acid (2) along with seven known ones, stigmast-5,28(29)-dien-3β-ol (3), β-sitosterol (4), carotene (5), fraxetin (6), p-coumaric acid (7), cis-p-hydroxycinnamic acid (8), ferulic acid (9) were obtained from the whole plant of Pachysandra terminalis. The structures of these compounds were elucidated by comprehensive spectroscopic methods including 1D, 2D NMR, MS, IR and ECD data analysis. Notably, compounds 3 and 5~8 were isolated from genus Pachysandra for the first time. Moreover, compounds 1~3 and 6~8 were tested their cytotoxic activities against three cancer cells, however, only compound 1 showed inhibitory effect in SW620 cells with IC50 value of 47.7 μM.
DOI http://doi.org/10.25135/rnp.346.2205.2465 Keywords Pachysandra terminalis chemical composition isolation and purification steroids fatty acids DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.11) Bioactive Alkaloids from the Beibu Gulf Coral-associated Fungus Acremonium sclerotigenum GXIMD 02501
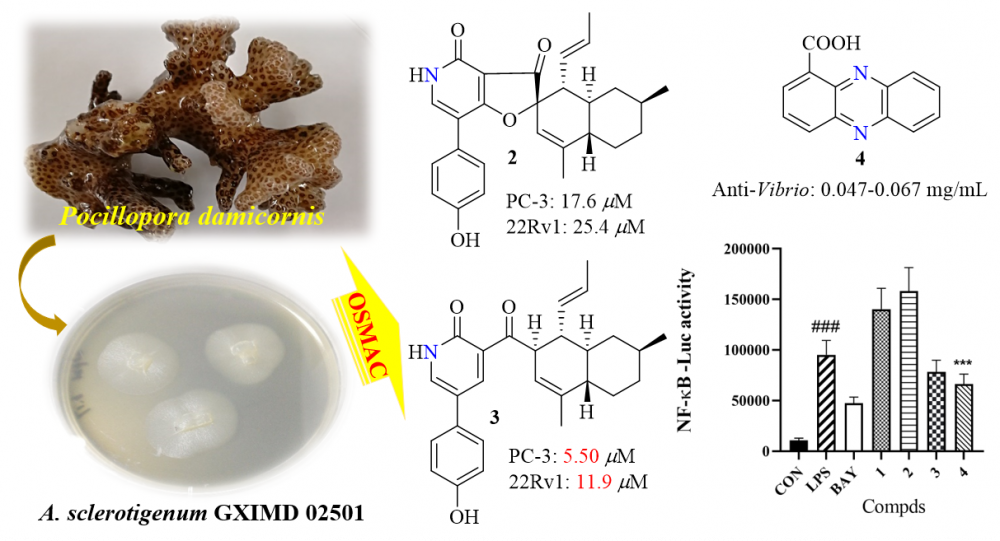
The Beibu Gulf represents an underexplored reservoir of marine micro-/organisms and secondary metabolites. Three uncommon 4-hydroxy-2-pyridone alkaloids and one phenazine alkaloid were obtained from the Beibu Gulf coral-associated fungus Acremonium sclerotigenum GXIMD 02501 via OSMAC approach. They were identified as campyridones D (1) and A (2), ilicicolin H (3), and phenazine-1-carboxylic acid (4), respectively, by spectroscopic analysis and comparison with literature values. All of them were evaluated for cytotoxic, anti-Vibrio, and NF-κB luciferase inhibitory activities. Compounds 2 and 3 showed cytotoxicity against two prostate cancer cell lines, with IC50 values of 17.6 ± 1.3 and 5.5 ± 1.2 μM for PC-3, while 25.4 ± 1.7 and 11.9 ± 1.3 μM for 22Rv1, respectively. Besides, compound 4 showed promising anti-Vibrio activity with MIC values of 0.047–0.067 mg/mL and also displayed inhibition of LPS-induced NF-κB activation at 10 μM.
DOI http://doi.org/10.25135/rnp.329.2204.2437 Keywords Acremonium sclerotigenum coral-associated fungi alkaloids bioactivity cytotoxicity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.12) Furan Derivatives and Amides from Elaeocarpus apiculatus
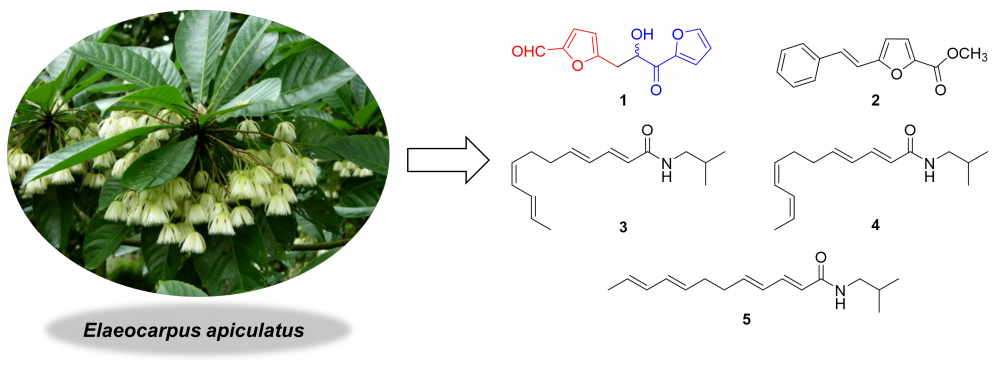
The first phytochemical study of Elaeocarpus apiculatus led to the isolation of five compounds, including two furan derivatives (1 and 2) and three amides (3-5). Among them, compound 1 was a new furan derivative, and compounds 2-5 were discovered from the genus Elaeocarpus for the first time. The characterization of these compounds was achieved by HRMS, UV, IR and NMR data. Compounds 1, 3 and 4 showed weak nitric oxide (NO) inhibitory effects. A potential biogenetic pathway for the formation of 1 was proposed.
DOI http://doi.org/10.25135/rnp.331.2203.2376 Keywords Elaeocarpus apiculatus Elaeocarpaceae furan derivative amide DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.13) Three New Chromone Derivatives from the Deep-Sea-Derived Fungus Penicillium thomii
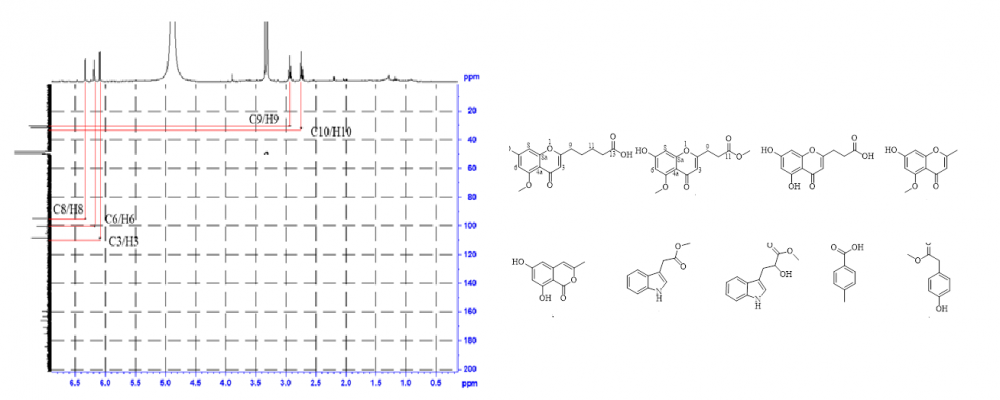
Chemical investigation of the EtOAc extract of a deep-sea-derived fungus Penicillium thomii Maire YPGA3 cultured on rice solid medium led to the isolation of three new chromone analogs, named penithochromones U-W (1-3), along with six known compounds (4-9). The structures were determined by extensive analyses of spectroscopic data (NMR and HRESIMS data). Compounds 1-3 are 5,7-dioxygenated chromone analogs bearing an aliphatic acid side chain. All isolated compounds were inactive toward the a-glucosidase at the concentration of 200 mM.
DOI http://doi.org/10.25135/rnp.330.2204.2433 Keywords Penicillium thomii; deep-sea fungus chromone derivative chromone derivative penithochromone DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.14) Psammosilenin C, a New Cyclic Peptide from Psammosilene tunicoides
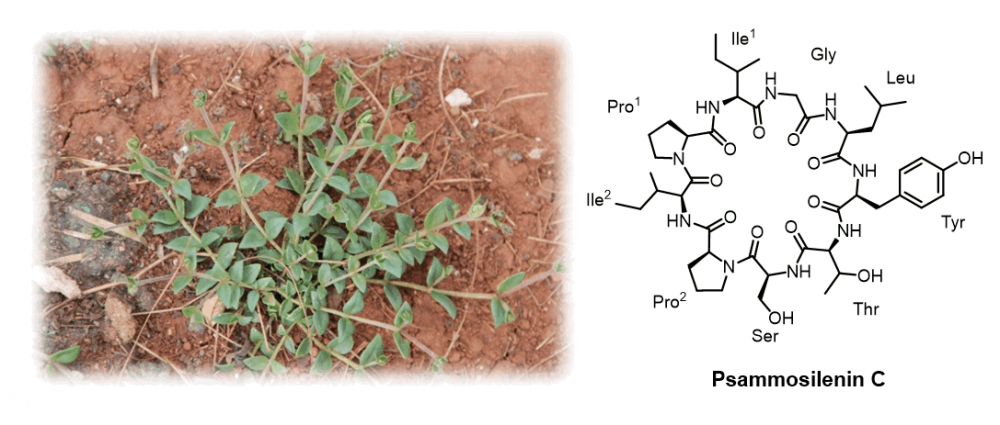
Phytochemical investigation of Psammosilene tunicoides led to isolation of a new cyclic peptide, psammosilenin C (1) and three known compounds, 1-O-(β-D-glucopyranosyl)-(2S,3R,4E,8E)-2-[(2R)-2-hydroxypentadecanoylamino]-4,8-octadecadiene-1,3-diol (2), dihydroferulic acid (3) and vanillylacetone (4). Their structures were elucidated by comprehensive spectroscopic methods, including 1D and 2D NMR spectroscopic, and HR-ESI-MS spectrometric data. Compounds 1, 2 and 4 showed inhibitory activities on lipopolysaccharide (LPS)-induced NO release in RAW 264.7 cells.
DOI http://doi.org/10.25135/rnp.336.2203.2399 Keywords Psammosilene tunicoides cyclic peptide anti-inflammatory activity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.15) A New Megastigmane Glycoside and Other Constituents from Amomum muricarpum Elmer
An updated phytochemical investigation of the aerial parts of Amomum muricarpum Elmer led to the isolation of a new megastigmane glycoside, (3S*,5R*,6R*,9R*)-6,9-epoxy-3,5-megastigmanediol 3-O-rutinoside (1) and five known phenolic compounds. Their structures were elucidated by spectroscopic evidence including HRESIMS and NMR data. All the isolates were reported for the first time from this plant. The anti-inflammatory effect via the inhibition of NO production of isolated compounds was evaluated in LPS-stimulated RAW 264.7 cells. Our study found that 5,7-dimethoxyflavone (4) was the most active compound with the IC50 = 29.5 µM.
DOI http://doi.org/10.25135/rnp.333.2203.2395 Keywords Amomum muricarpum megastigmane 5,7-dimethoxyflavone anti-inflammation DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.16) A New Lignan from Leaves of Ormosia xylocarpa
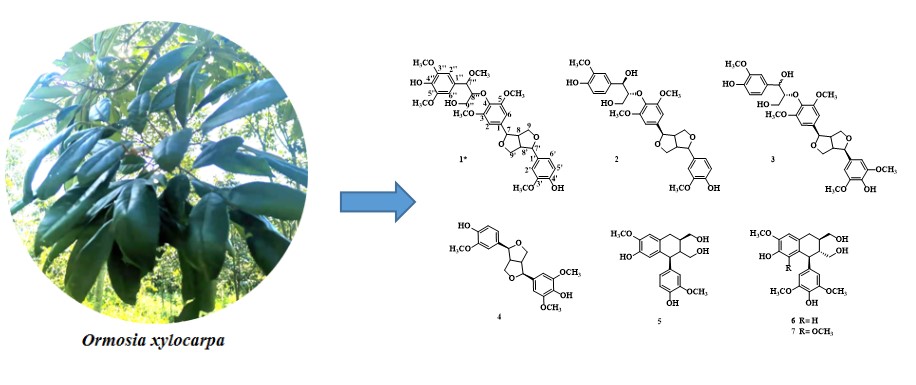
A new lignan, 4,4²-dihydroxy-3,3¢,5¢,3²,5²,7²-pentamethoxy-7,9¢;7¢,9-diepoxy-4¢,8²-oxy-8,8¢-sesquineo-lignan-propanol (1), along with six known lignans (2-7) was isolated from the leaves of Ormosia xylocarpa (Chun ex L. Chen). The structure of compound 1 was elucidated through comprehensive 1D and 2D NMR, UV, IR, and HRMS analyses. Compounds 2~3 performed strong antioxidant activity, the median clearance concentration of DPPH, ABST+, and ·OH was lower than 40 μM.
DOI http://doi.org/10.25135/rnp.338.2203.2386 Keywords Ormosia xylocarpa lignans chemical constituents antioxidant activity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.17) Lactones from Glomerella cingulata Cultivated in Rice:Structural Studies and Antimicrobial Evaluation
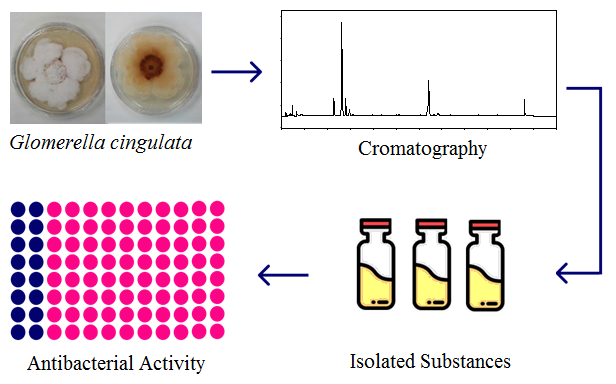
Extracts of the endophytic fungus Glomerella cingulata grown in polish rice, were fractionated by chromatographic procedures including preparative scale HPLC. The isolated compounds were structurally elucidated through spectroscopic analysis, mainly 1H and 13C NMR and HRMS. These analyzes allowed the identification of pestalotin-1 (1) and the phthalides (3R*,8S*)-5,7-dihydroxy-3-(1-hydroxyethyl)-phthalide (2) and (3R*,8R*)-5,7-dihydroxy-3-(1-hydroxyethyl)-phthalide (3). The extracts, their fractions and isolated substances were tested against several bacteria. The lactone 2 showed some activity against lineages of E. coli and Enterococcus faecalis while its diastereoisomer 3 and pestalotin-1 were inactive.
DOI http://doi.org/10.25135/rnp.340.2202.2352 Keywords Endophytic fungus Glomerella cingulata antimicrobial activity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.